Paramagnetism
In addition to diamagnetism and ferromagnetism, there is another, special form of magnetism: the so-called Paramagnetism. These substances have no measurable magnetization without an external magnetic field. However, when an external magnetic field is introduced, the material magnetizes noticeably and intensifies its internal magnetic field. Typical examples of Paramagnetism from chemistry are liquid oxygen, platinum or chromium.
How does it become Paramagnetism?
Paramagnets by themselves have no magnetic order, so no uniform orientation of the magnetic moments. When the external magnetic field is removed, the generated alignment is resolved again because the magnetic moments are randomly aligned due to thermal movements.
Physical view of Paramagnetism
The common feature of paramagnetic substances is that their magnetic permeability μ_r always greater than 1 and the susceptibility is
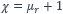
The greater the susceptibility of a substance, the easier it is to magnetize it. This size of the magnetisability of matter is logically closely related to the property of how matter behaves in an external magnetic field. The magnetization M of a substance is proportional to the external magnetic field H:

On the one hand, paramagnets have unpaired electrons, but on the other hand, atoms with magnetic moments, in the cause lies in the electron spin. The magnetic moments align with the action of an external magnetic field in the direction of the magnetic field lines, but always remain in their position - comparable to many small fixed rod magnets.
Temperature dependence in Paramagnetism
At room temperature, the magnetic moments are arbitrarily aligned because the heat energy is greater than the energy that would be required to flip the electron spins. Interestingly, however, paramagnets at low temperatures can attain a certain order state even without an external magnetic field.
On the other hand, the ferromagnets are the ones that exhibit paramagnetic behavior above their Curie temperature. Such phenomena are described by so-called phase transitions (thermodynamic transitions). It follows that the magnetic susceptibility of paramagnets according to the Curie constant is inversely proportional to the temperature.
 Thousands of products in stock
Thousands of products in stock