Curie temperature
The eponym of the so-called Curie temperature is the French physicist Pierre Curie. The Curie temperature is substance-specific. Above the Curie temperature, the magnetic properties of a substance change. Iron, for example, is no longer attracted to any magnet above the Curie temperature. The Curie temperature for nickel is 358 ° C, for iron 768 ° C and for cobalt 1127 ° C.
More precise definition of the Curie temperature
The temperature at which a ferromagnetic becomes the paramagnet is called the Curie temperature. It is often mentioned in the context of remanence: Above the substance-specific Curie temperature this remanence of a ferromagnetic substance disappears. The Curie temperature indicates the temperature at which a magnet must be heated in order to demagnetize it.
Remanence and magnetization, Curie temperature
For a better understanding of the effect, here is an explanation of remanence: When a ferromagnetic material is exposed to a magnetic field, it becomes magnetized. The material becomes magnetic and remains so even when the external magnetic field no longer exists. This residual magnetization is called remanence. The magnetization itself is accomplished by electron spin. The magnetic moments of the spins align with the magnetic field and are stabilized in the material by the so-called exchange interaction. It is this exchange interaction that prevents the atomic movement (ie the thermal energy of the substance) from destroying the alignment again.
In this context it is only logical that by increasing the thermal energy a point can be reached, from which the exchange interaction is overcome. As the temperature increases, the orientation of the electron spins shifts in large areas simultaneously. This shift is called a Barkhausen jump.
Wide areas of the electron spin thus remain aligned parallel, these are the so-called Weißschen districts. Accordingly, with each Barkhausen jump, a new Weißscher range forms - until the thermal energy is greater than the energy of the exchange interaction. At this time, the magnetic moments, causing the common orientation is completely lost. From now on, the material is a paramagnet.
What was another Paramagnet?
Contrary to a ferromagnet, a paramagnetic material demagnetizes immediately after the external field has been turned off.
So if an outer field is not there, the electron spins are completely random. However, as long as the external field is applied, it is amplified by a paramagnet. This gain decreases as the temperature increases, and more and more energy is needed to align the spins. The so-called Curie-Weiss law describes the dependence of the magnetic susceptibility X of a material on the temperature T:
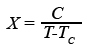 Here, C is the so-called Curie constant, which of course is also material-specific. The law shown (1) was first formulated by the physicist Pierre Curie in 1896. It was further developed by the French physicist Pierre-Ernest Weiss in 1907 - hence the double naming.
Here, C is the so-called Curie constant, which of course is also material-specific. The law shown (1) was first formulated by the physicist Pierre Curie in 1896. It was further developed by the French physicist Pierre-Ernest Weiss in 1907 - hence the double naming.
 Thousands of products in stock
Thousands of products in stock